Revolutionizing healthcare & pharma with real-world evidence


Kaolel is a California based company focused solely on the life science industry.
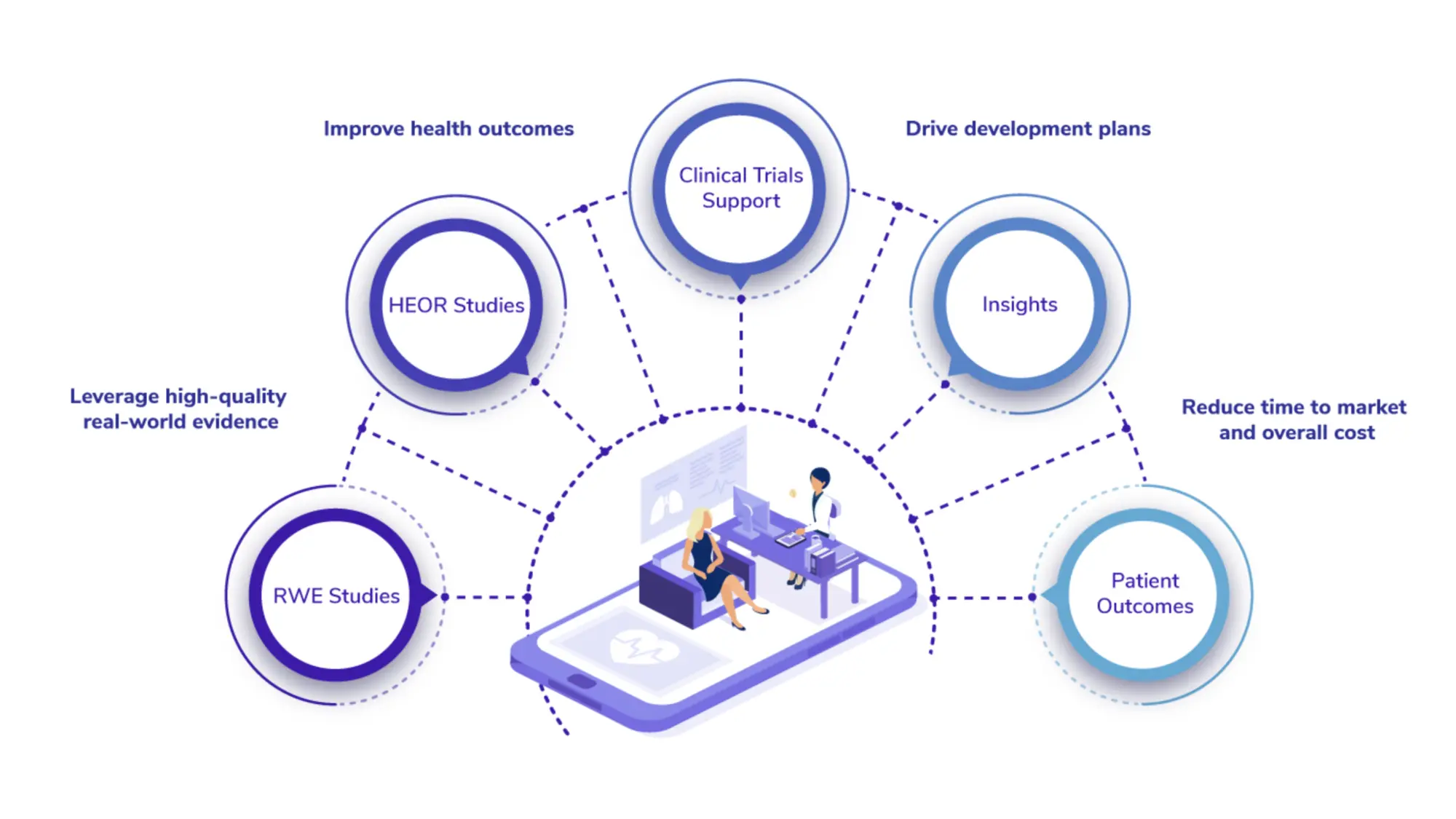
We are deeply embedded in real world evidence generation at all stages of development and commercialization of drugs and medical devices. Our core strength as an evidence-based platform is our unmatched solutions in characterizing real-world use, and clinical and economic value of medical products and devices.
We are a proven partner with solid knowledge in epidemiology methods, biostatistical analysis, data science and health economics. We work with our clients to lighten the burden of proof and communicate the real-world value of their medical product to meet the needs of their stakeholders.
We invite you to leverage our platform to accelerate product time to market, reduce development costs, and innovate with the power of real world data and artificial intelligence to stay ahead of the curve.
Who we partner & collaborate with
Kaolel offers a full range of real-world evidence services in strategy, consulting, study execution and technology that help deliver the best outcomes for patients. We work with stakeholders in Pharmaceutical, Biotechnology and medical device companies across the globe.
Research & Development
- Electronic health records
- Insurance claims
- Patient registries
- And more